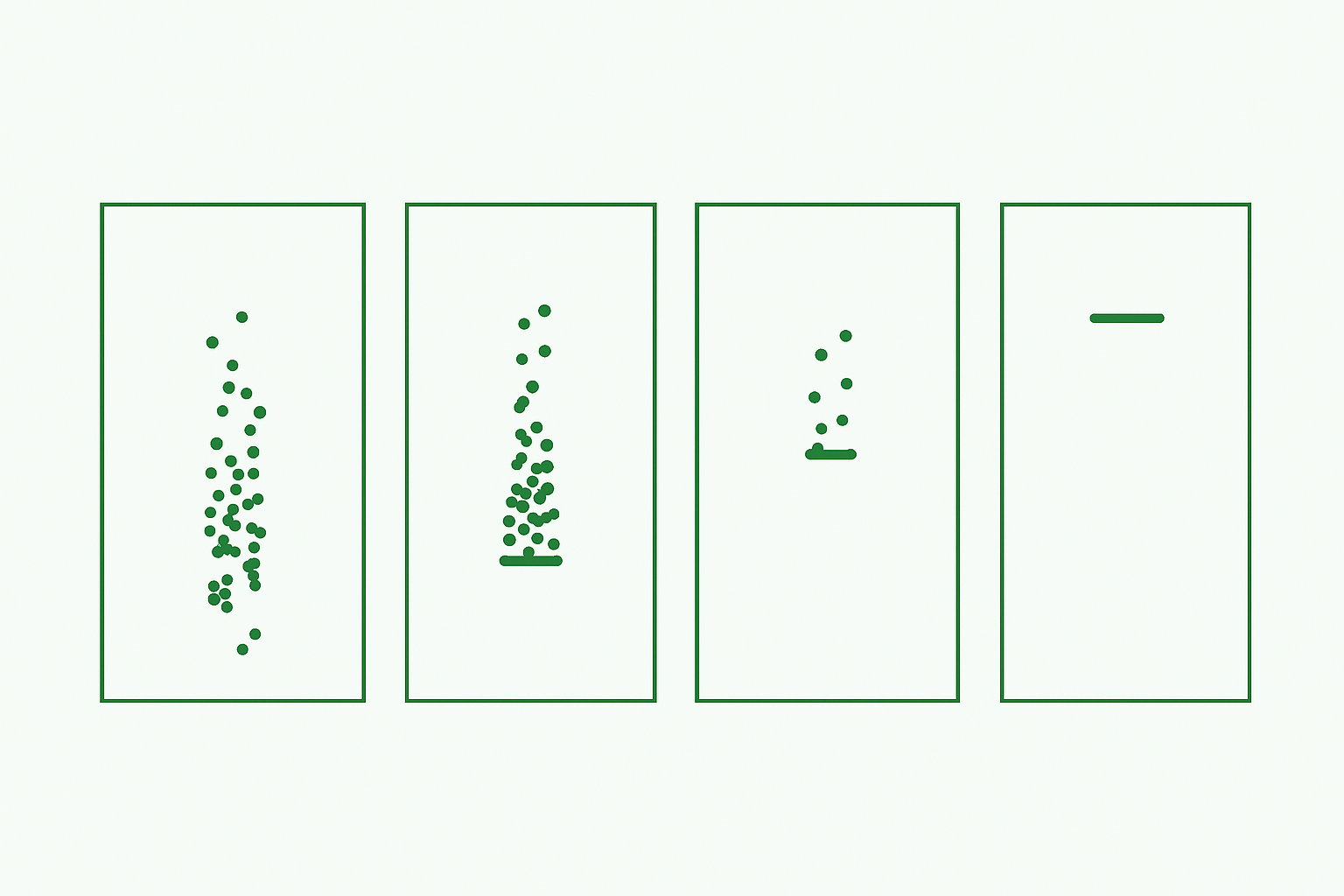
One can think of the life of a bioassay in two main acts. First comes the design and development of the assay, where the assay method is conceptualised, refined,... read more →
One common use for bioassays is to test manufactured biologic products before they are released to the wider consumer market. While all bioassays used for this purpose will have... read more →
Statistics is hard, words are harder. So often when working through the statistics behind a bioassay, the correct path is obscured behind a word salad of jargon placed seemingly... read more →
In many fields of science, researchers often face the challenge of measuring extremely small quantities. The life sciences are no exception, with experiments regularly requiring accurate and precise detections... read more →
In In Vitro vs In Vivo Potency Assays: Modern Vaccine Batch Release, we examined the pros and cons of two methods of testing vaccine potency: in vivo assays, which... read more →